In collaboration with the Belfer Center for Applied Cancer Science, and the Brigham and Women’s Hospital’s Pathology Department, we develop novel complementary approaches to predict patients’ responses to targeted therapies and therapeutic combinations.
The primary objective is to arrive at a treatment rationale well before the necessary information becomes available from standard diagnostic methods. The exploitation of patient-derived models established in our lab is a key component of these efforts.
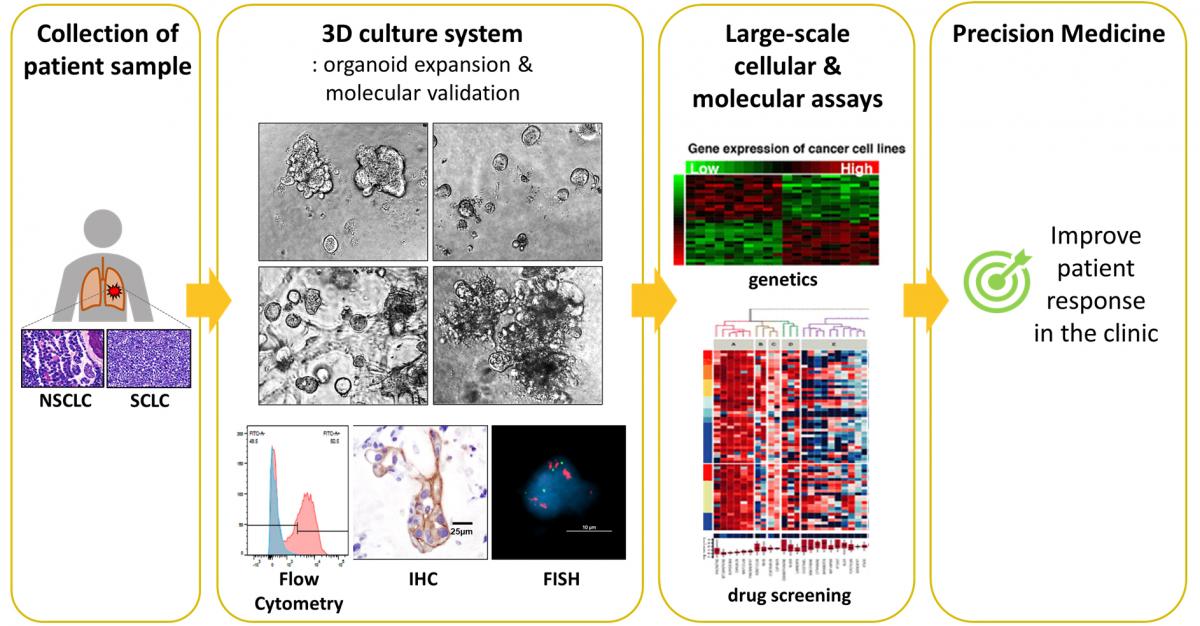
Organoid platform for drug testing
Cancer cells harvested from patients’ tumor biopsies have an inherent ability to propagate and self-organize in vitro into viable 3D structures, termed organoids. Because of their genetic/epigenetic fidelity to the original tumor and their 3D arrangement more closely emulating physiological conditions, they are a suitable platform for early exploratory studies that would be impractical to perform in vivo.
Organoids are routinely grown in 364-well plates, a format that is amenable to high-throughput screens designed to identify effective drug candidates or novel targets. Because of their intrinsic features, low cost and relative ease of manipulation, organoids have become an efficient tool in our lab for identifying patient-specific therapeutic approaches, and more generally translating our pre-clinical knowledge into novel therapies.
IncuCyte imaging
In collaboration with the Belfer Center for Applied Cancer Science, and the Brigham and Women’s Hospital’s Pathology Department, we develop novel complementary approaches to predict patients’ responses to targeted therapies and therapeutic combinations.
The primary objective is to arrive at a treatment rationale well before the necessary information becomes available from standard diagnostic methods. The exploitation of patient-derived models established in our lab is a key component of these efforts.
High throughput screening
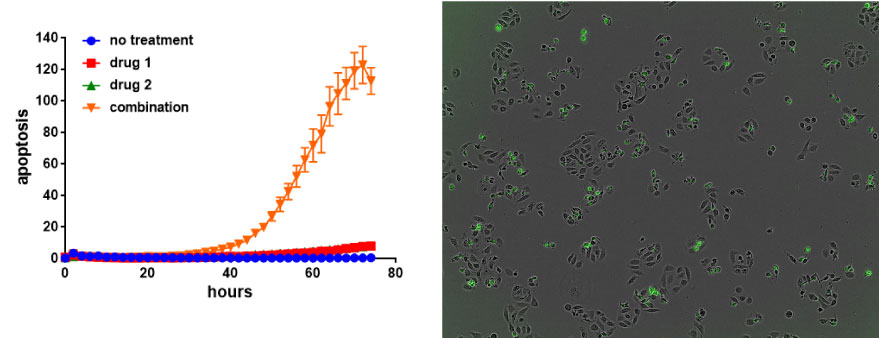
In our effort to identify new actionable targets in naïve or drug resistant lung cancer, and subsequently develop novel effective treatments, high throughput screening capability has become an essential component of our research. Whether exploiting the CRISPR/Cas9 technologies, single cell RNAseq, or drug libraries build with our collaborators, we actively search for and have been able to establish novel candidate gene targets, gene signatures, and compounds, all of which can potentially be translated to improved clinical outcomes for patients.
In addition to the IncuCyte, we use the HP drug printer and other high-throughput tools to screen for new compounds synthesized by our collaborators that may show better or complementary efficacy against various lung cancer genotypes. In addition, we typically survey combinations of both new and established drugs for possible synergies.
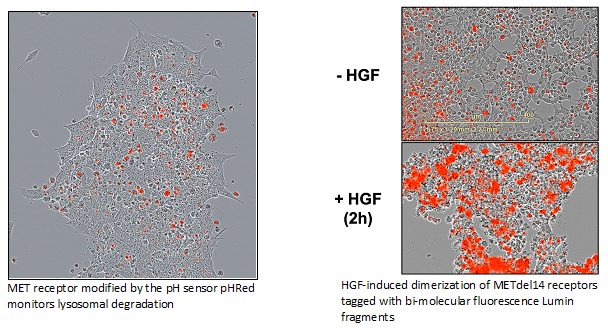
Development of imaging methods and sensors
Understanding the basic biology of wild type or mutant proteins – how they contribute to tumorigenicity and/or drug resistance – is requisite to all targeted therapies. Yet, our knowledge of many cellular processes involving such proteins remains inchoate. One of our lab’s goals is to develop imaging tools that may aid in elucidating cellular processes involved in subcellular localization, receptor dimerization, complex formation, cycling, or degradation. To this end, fluorescent systems are constructed in ways amenable to easy and scalable readout.